Master Faraday’s law numericals.
Featured Articles on: VIVA QUESTION
Cell and Internal Resistance Numericals for IIT JEE
Boost problem solving on cells.
Problems on resistance, circuits, and current distribution.
Strengthen circuit analysis.
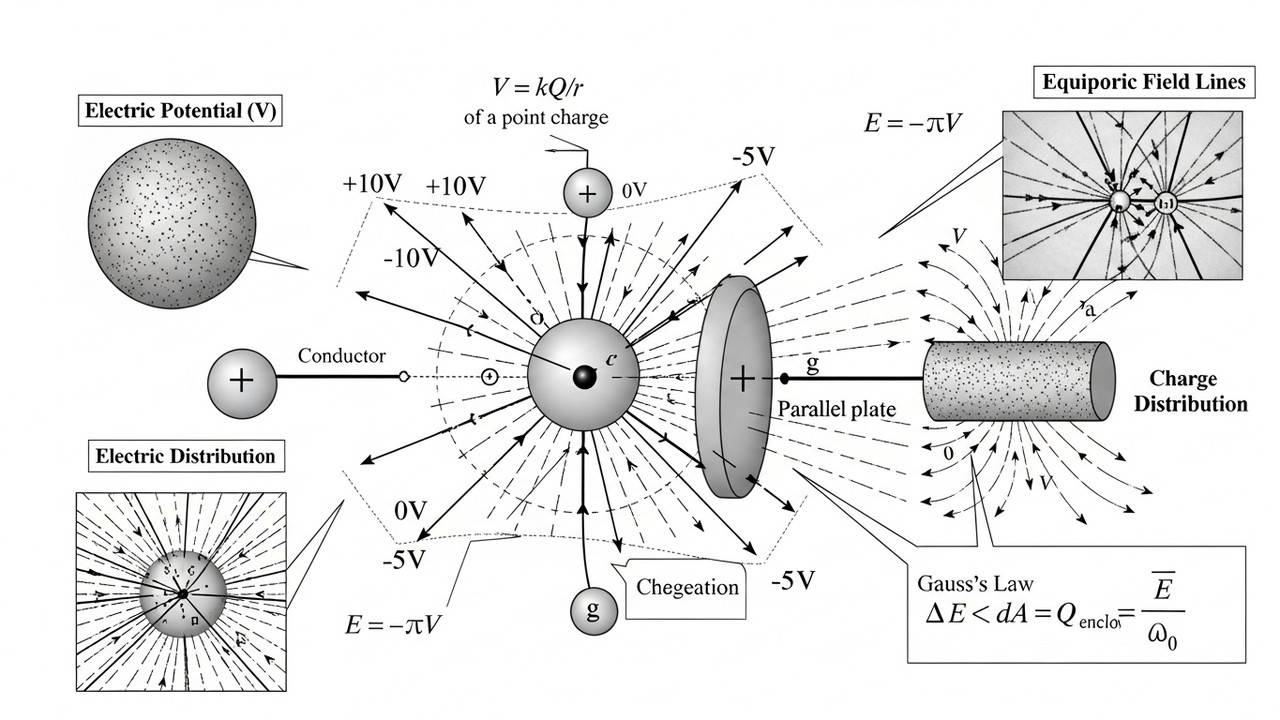
Electric Potential Numericals for IIT JEE
Sharpen potential-based problem solving.
Momentum Mastery Quiz: From Fundamentals to Advanced Concepts
This Momentum Mastery Quiz is designed to assess and enhance your comprehensive understanding of momentum in physics. Featuring 25 multiple-choice questions, the quiz covers a wide range of topics from basic definitions and calculations of linear momentum to more advanc…
Navigating the CBSE Board Exams 2025: A Comprehensive Guide
Conquer the CBSE Board Exams 2025 with our guide! Learn effective study strategies, time management tips, and overcome exam anxiety for success.
Metal compound A reacts with dilute hydrochloric acid to produce effervescence. The gas evolved extinguishes a burning candle. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride. Also, determine the Metal Compound A.
Based on the information provided, the metal compound A reacts with dilute hydrochloric acid to produce effervescence, and the gas evolved extinguishes a burning candle. Additionally, one of the compounds formed in the reaction is calcium chloride. Let's write the...
Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
When an acid reacts with a metal, hydrogen gas (H2) is usually liberated. The reaction between an acid and a metal is a type of single-displacement or single-replacement reaction, where the more reactive metal displaces hydrogen from the acid, forming a metal salt and...
Why should curd and sour substances not be kept in brass and copper vessels?
Curd and sour substances should not be kept in brass and copper vessels because these metals can react with acidic foods, leading to potential health hazards. The main concern is the leaching of toxic metals into the food or liquid being stored, which can contaminate...
10 Examples of combination reactions
1. The reaction between hydrogen gas and oxygen gas to form water: 2H2 + O2 → 2H2O 2. The reaction between iron and sulfur to form iron sulfide: Fe + S → FeS 3. The reaction between magnesium and oxygen to form magnesium oxide: 2Mg + O2 → 2MgO 4. The reaction between...