Cell division is fundamental to life, and the process is carefully controlled. Cell cycle regulation is a critical area of study, as errors in this control can lead to severe consequences, like cancer. This article explores the mechanisms of cell division and the critical roles of checkpoints and regulatory proteins.
On This Page
Read More
Cell division, the fundamental process driving growth and repair in all living organisms, is a tightly controlled process. The fidelity of this process is paramount; errors can lead to severe consequences, including diseases like cancer. Understanding the intricacies of this control, especially the role of cell cycle regulation, is therefore crucial.
Unveiling the Core of Cell Division: The Cell Cycle
Embark on a journey into the heart of cellular life, where the cell cycle regulation orchestrates the symphony of division. This cycle, a carefully choreographed series of events, ensures that each daughter cell receives a complete and accurate copy of the parent cell's genetic material. The process is not merely a simple split; it is a complex interplay of molecular signals, checkpoints, and regulatory proteins.
The Phases of Division: A Detailed Look
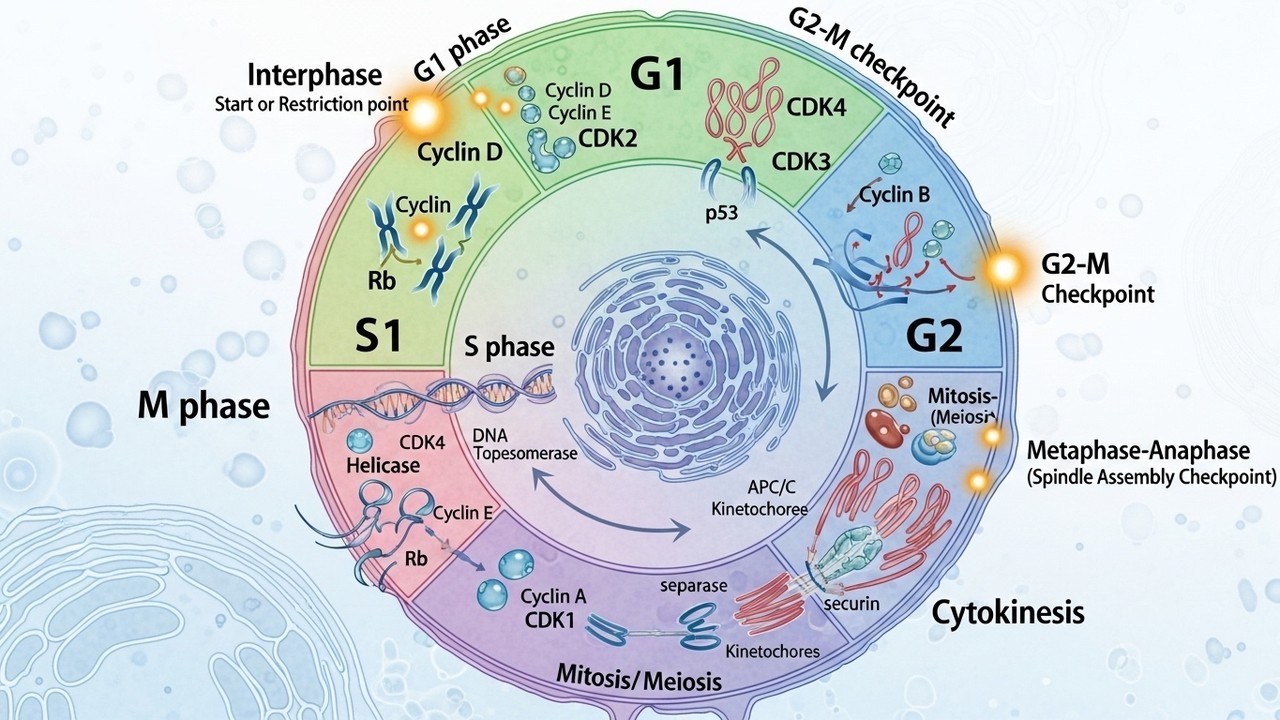
The cell cycle is divided into distinct phases: G1, S, G2, and M. G1, or Gap 1, is a period of growth where the cell prepares for DNA replication. The S phase is where DNA synthesis occurs, duplicating the genetic material. G2, or Gap 2, is a second growth phase where the cell prepares for mitosis. The M phase, or mitosis, is the actual division phase, where the duplicated chromosomes separate and the cell divides into two daughter cells. The entire process, which can take anywhere from 10 to 20 hours, is a testament to the complexity and precision of cellular biology.
Each phase is a critical step in the cell cycle, with its own set of checks and balances. The cell must ensure that all necessary components are in place before proceeding to the next phase. Disruptions at any stage can have serious consequences, potentially leading to genomic instability and the development of diseases. The precise timing and coordination of these phases are key to maintaining cellular health and preventing uncontrolled growth.
Consider the analogy of an assembly line. Each stage must be completed correctly before the product moves to the next. Similarly, each phase of the cell cycle must be completed correctly before the cell proceeds. This analogy highlights the need for precise control and the potential for problems when this control is lost. The cell cycle is not a linear process; it is a carefully orchestrated series of events.
Checkpoints: The Gatekeepers of the Cell Cycle
The cell cycle features critical checkpoints that act as surveillance mechanisms, ensuring the accuracy and integrity of the division process. These checkpoints are strategically positioned at key points within the cycle, such as the G1/S, G2/M, and metaphase checkpoints. They monitor specific cellular conditions, such as DNA damage, incomplete replication, and chromosome alignment. If any errors are detected, the checkpoint mechanisms halt the cycle, allowing the cell to repair the damage or correct the issue before proceeding.
The G1/S checkpoint, for example, assesses the cell's readiness to replicate its DNA. If DNA damage is detected, the cell may undergo repair or initiate programmed cell death (apoptosis) to prevent the propagation of errors. The G2/M checkpoint ensures that DNA replication is complete and that the DNA is not damaged before the cell enters mitosis. The metaphase checkpoint monitors chromosome alignment on the mitotic spindle, ensuring that each daughter cell receives the correct number of chromosomes. These checkpoints are essential for maintaining genomic stability and preventing the development of diseases.
These checkpoints are not passive observers; they actively respond to cellular conditions. When a problem is detected, the checkpoint mechanisms activate signaling pathways that can halt the cell cycle. This pause allows the cell time to repair the damage or correct the error. If the damage is irreparable, the checkpoint mechanisms can trigger apoptosis, preventing the cell from dividing and potentially causing harm. This active response highlights the dynamic nature of cell cycle regulation.
Molecular Players: Cyclins and CDKs
The regulation of the cell cycle is a complex process involving several key molecular players, most notably cyclins and cyclin-dependent kinases (CDKs). These proteins work together to orchestrate the transitions between the different phases of the cell cycle. Cyclins act as regulatory subunits, while CDKs are the catalytic enzymes that drive the cycle forward. The interaction between cyclins and CDKs is carefully controlled, ensuring that the cycle progresses in an orderly manner.
Cyclins: The Orchestrators
Cyclins are a family of proteins whose levels fluctuate throughout the cell cycle. They bind to and activate CDKs, the enzymes that drive the cell cycle. The specific cyclin present in the cell determines which CDK is activated and, consequently, which phase of the cell cycle is promoted. For example, cyclin D is active in G1 phase. Cyclin E is important for the G1/S transition. Cyclin A plays a role in the S phase. Cyclin B is essential for the G2/M transition. The periodic expression of cyclins is a key mechanism for controlling the cell cycle.
The levels of cyclins rise and fall in a cyclical manner, reflecting their roles in the progression of the cell cycle. The levels of cyclin rise as the cell progresses through a certain phase. Once the cell reaches the next phase, the cyclin levels decrease. This cyclical pattern ensures that CDKs are activated at the appropriate times, driving the cell cycle forward. The synthesis and degradation of cyclins are tightly regulated, ensuring that the cell cycle progresses in an orderly manner. The precise timing of cyclin expression is critical for proper cell division.
Consider the example of a conductor leading an orchestra. The conductor, in this case, would be the cyclin. The orchestra members would be the CDKs. The conductor signals the orchestra members to play at the right time, which is similar to how cyclins activate CDKs. The music, in this case, is cell division. The cyclical expression of cyclins ensures that the music is played at the right time, ensuring the smooth progression of the cell cycle. The conductor's role is essential to the smooth progression of a musical performance.
CDKs: The Engines
Cyclin-dependent kinases (CDKs) are a family of protein kinases that are activated by binding to cyclins. They phosphorylate (add a phosphate group to) other proteins, which in turn regulate the cell cycle. The activity of CDKs is tightly controlled by various mechanisms, including cyclin binding, phosphorylation, and dephosphorylation. These mechanisms ensure that CDKs are activated only at the appropriate times, promoting the orderly progression of the cell cycle.
The CDKs are the engines that drive the cell cycle forward. They phosphorylate proteins, which then trigger the events of each phase. For example, CDK1, complexed with cyclin B, promotes entry into mitosis. CDK2, complexed with cyclin E, helps to initiate DNA replication. The phosphorylation of these proteins is a critical step in the cell cycle. The CDKs are the engines that drive the cell cycle forward.
The analogy of a car engine is useful here. The CDKs are like the engine, and the cyclins are like the key. The key is needed to start the engine, and the cyclin is needed to activate the CDK. Once the engine is running, it can drive the car forward. The CDKs, once activated, drive the cell cycle forward. The car engine analogy helps understand the role of CDKs in the cell cycle.
Dysregulation and Disease: The Cancer Connection
When the intricate mechanisms of cell cycle regulation go awry, the consequences can be dire. Errors in this regulation can lead to uncontrolled cell division, a hallmark of cancer. Mutations in genes that control the cell cycle, such as those encoding cyclins, CDKs, or checkpoint proteins, can disrupt the delicate balance and lead to the formation of tumors. The study of these dysregulations is therefore crucial for understanding the development and progression of cancer.
Tumor Suppressors: Guardians of the Genome
Tumor suppressor genes, such as p53 and Rb, play a critical role in cell cycle regulation. They act as gatekeepers, preventing cells with damaged DNA from dividing. When these genes are mutated or inactivated, the cell cycle checkpoints can be bypassed, allowing damaged cells to proliferate and potentially form tumors. The function of tumor suppressor genes is analogous to a brake pedal in a car; when functioning correctly, they can stop the cell cycle when necessary.
The p53 gene, often referred to as the "guardian of the genome", is a key regulator of the cell cycle. It is activated in response to DNA damage and can initiate cell cycle arrest, DNA repair, or apoptosis. When p53 is mutated, it loses its ability to perform these functions, and the cell can continue to divide even with damaged DNA. This can lead to the accumulation of mutations and the development of cancer. The p53 gene is one of the most frequently mutated genes in human cancers.
The Rb gene, another important tumor suppressor gene, regulates the G1/S transition. It prevents the cell from entering the S phase until it is ready. When Rb is inactivated, the cell can bypass the G1/S checkpoint and enter the S phase prematurely. This can lead to uncontrolled cell division and tumor formation. The Rb gene is frequently inactivated in retinoblastoma, a type of eye cancer. The study of tumor suppressor genes is essential for understanding the development of cancer.
Targeted Therapies: Restoring Control
The study of cell cycle regulation has paved the way for the development of targeted cancer therapies. These therapies aim to specifically disrupt the pathways that are dysregulated in cancer cells, thereby restoring controlled cell division. CDK inhibitors, for example, are designed to block the activity of CDKs, preventing cancer cells from dividing. Checkpoint inhibitors are designed to reactivate the immune system to identify and destroy cancer cells.
The development of CDK inhibitors has been a significant breakthrough in cancer treatment. These drugs specifically target CDKs that are overactive in cancer cells, preventing them from driving the cell cycle forward. This can lead to cell cycle arrest and apoptosis. The effectiveness of CDK inhibitors varies depending on the type of cancer and the specific CDK that is targeted. CDK inhibitors are used in the treatment of breast cancer, lung cancer, and other types of cancer.
The use of checkpoint inhibitors is another promising approach in cancer therapy. These drugs block the checkpoint proteins that are used by cancer cells to evade the immune system. This allows the immune system to recognize and destroy cancer cells. Checkpoint inhibitors have shown remarkable results in the treatment of several types of cancer. The study of cell cycle regulation continues to drive innovation in cancer treatment.
Key Takeaways
The cell cycle is a finely tuned process, and the regulation of this cycle is essential for maintaining cellular health. The checkpoints, cyclins, and CDKs are the key players in this intricate dance. Understanding the nuances of this regulation is critical for developing effective treatments for diseases like cancer. Continued research in this field promises to yield even more targeted and effective therapies in the future.
| Component | Function | Role in Cell Cycle |
|---|---|---|
| Cyclins | Regulatory proteins | Activate CDKs, driving the cycle |
| CDKs | Protein kinases | Phosphorylate proteins, regulating cycle phases |
| Checkpoints | Surveillance mechanisms | Ensure accuracy, halt cycle if errors detected |
We also Published
RESOURCES
- Cell cycle regulation: p53-p21-RB signaling
- Cyclin-dependent protein kinases and cell cycle regulation in ...
- Regulation of the cell cycle (article) | Khan Academy
- Dissecting the cell cycle regulation, DNA damage sensitivity and ...
- Cell cycle regulators (article) | Khan Academy
- Cell cycle regulation in hematopoietic stem cells - PMC
- Cell cycle regulation of human DNA repair and chromatin ...
- Cell cycle regulation: p53-p21-RB signaling - PMC
- Cell cycle - Wikipedia
- Cell Cycle Regulation by Checkpoints - PMC






3 Comments