Discover the science of enzymatic plastic upcycling. Learn how engineered hydrolases enable infinite circularity for mixed polymers, disrupting traditional mechanical recycling methods.
Featured Articles on: CHEMISTRY
Electrochemical Ammonia: Decarbonizing the Global Food Chain
A technical deep dive into electrochemical ammonia synthesis. Learn about ruthenium catalysts, modular production, and the decarbonization of the global food chain.
Air-to-Plastic: The Direct Air Capture (DAC) Chemical Feedstock Shift
Discover how Direct Air Capture (DAC) is redefining the chemical industry. Learn about the thermodynamics, catalytic conversion of CO2 to ethylene, and the rise of ‘Sky-Polyethylene’.
Bio-Cementation: The Chemical Industry Decarbonizes Construction
Discover the technical shift toward bio-cementation. Learn about MICP, urease enzymes, and the chemical pathways that allow the construction industry to sequester carbon in urban environments.
The Sodium-Glass Breakthrough: Chemistry Ends the Lithium Dependency
Discover how sodium-glass batteries are replacing lithium. Learn about the chemistry, geopolitical impact, and the future of solid-state energy storage through 15 technical illustrations.
Space Chemistry: Refining Lunar Regolith for On-Orbit Manufacturing
Discover the science of lunar regolith refining. This guide covers chemical extraction, molten salt electrolysis, and the future of on-orbit manufacturing and ISRU.
The Critical Role of the Top 5 Metals Used in Metallurgy Today
Explore the foundational elements of modern industry by delving into the top 5 metals used in metallurgy today, examining their unique properties, complex extraction and processing methods, and indispensable applications across various sectors. This comprehensive articl…
The Arrhenius Equation: Decoding Reaction Rates and Temperature Dependence
A comprehensive technical exploration of the Arrhenius Equation, its mathematical derivation, physical interpretation, and applications in chemical kinetics and thermodynamics.
Understanding Chemical Kinetics: A Comprehensive Guide
Explore the fundamentals of chemical kinetics, including rate laws, integrated rate equations, half-life calculations, and the Arrhenius equation. Essential for competitive exams.
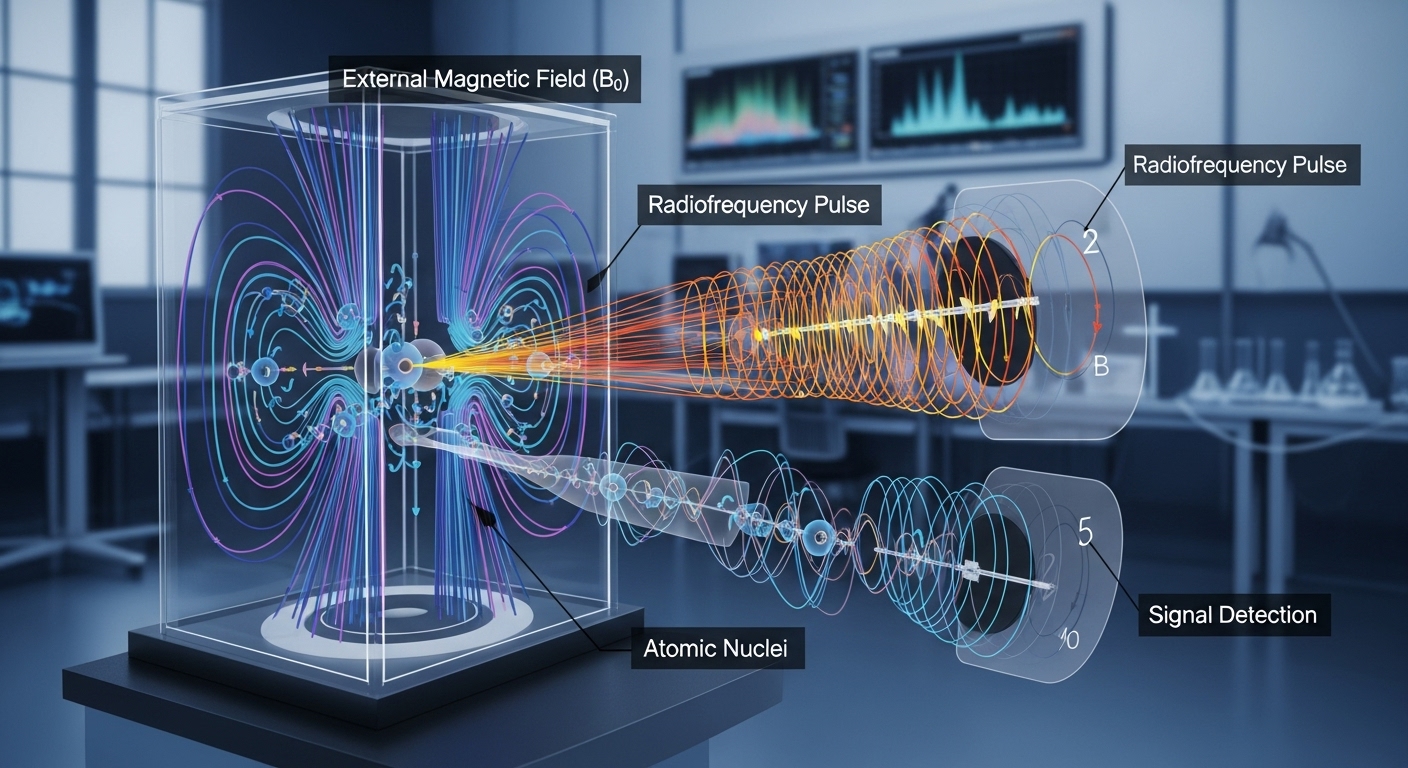
Understanding Nuclear Magnetic Resonance (NMR): A Deep Dive into Principles and Applications
Explore the fundamental principles of Nuclear Magnetic Resonance (NMR) spectroscopy, from nuclear spin and Larmor precession to chemical shift and relaxation processes. Discover its diverse applications in chemistry, physics, and medicine.
The Chemistry of Chromatic Instability: Why Synthetic Emerald Green Pigments Degrade
An in-depth technical analysis of the degradation mechanisms in copper acetoarsenite pigments. Explores thermodynamics, reaction kinetics, and spectroscopic evidence.
Rutherford Atomic Model: How Scattering Revealed The Nuclear Atom
Rutherford atomic model explains the nuclear structure of the atom with a bold claim: nearly all mass and positive charge reside in an extremely small nucleus while electrons occupy the surrounding space. Proposed after the famous gold-foil experiment, Rutherford...
Ideal Gas Law Application: real-world problem-solving for gases
Explore ideal gas law application, solving a problem that determines the volume of a gas under specific conditions of pressure and temperature.
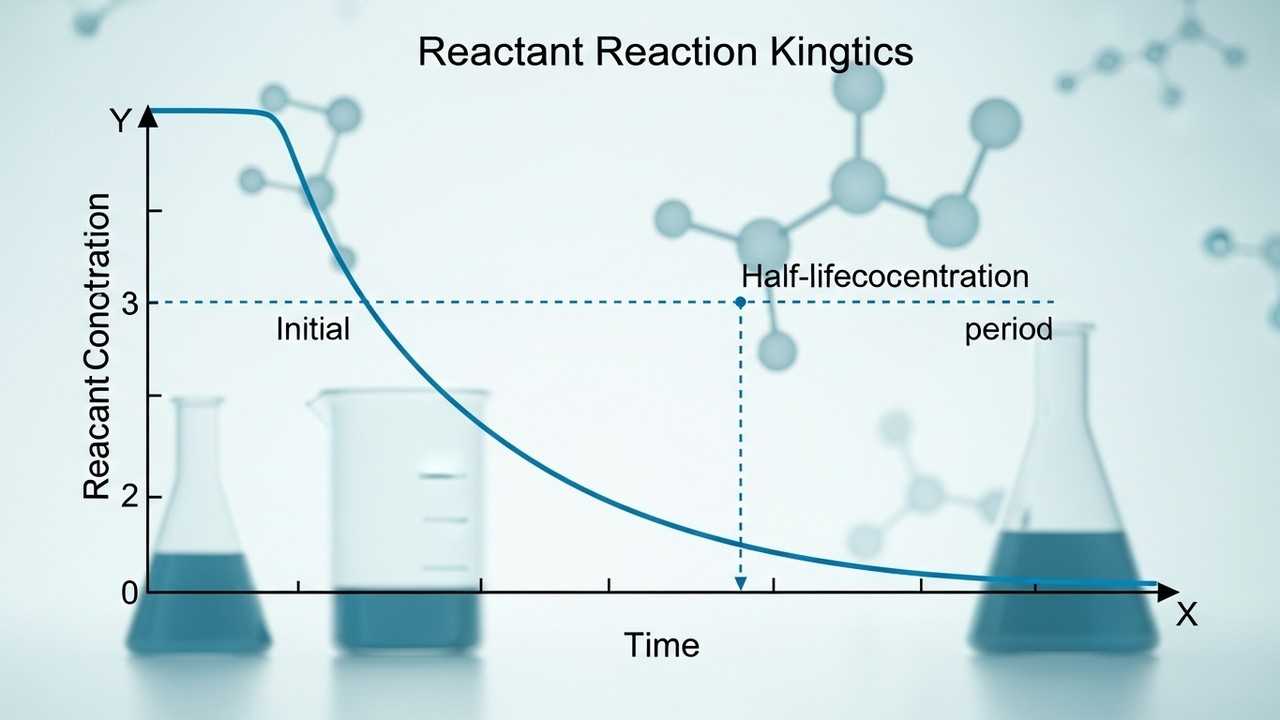
First-Order Reaction Half-Life: Calculating Reactant Remaining
Understand the concept of **First-Order Reaction Half-Life** and how to calculate the fraction of reactant remaining after a given time. This post will describe the half-life and explain how it impacts the decay of a chemical reaction.
Penguin Guano’s Surprising Impact on Antarctic Climate
New research reveals that penguin guano in Antarctica plays a significant role in climate cooling. Learn how penguin poop affects cloud formation.
New Study Challenges Thermodynamics Laws: Surprising Findings
A new study challenges the laws of thermodynamics revealing unexpected results in liquid mixtures. Learn more about the surprising findings!